Nutrition 330 Introductory Nutrition
Study Guide: Unit 12
Trace Minerals and Nutrients for Blood Health
Although present in only minute amounts in the body (less than 0.01% of body weight), trace minerals are essential for many vital functions. The essentiality of such small quantities is often difficult to determine; however, the development of highly sensitive instruments has enabled investigators to analyze food and tissue samples for extremely low concentrations, and has allowed for a clearer understanding of the role of trace minerals.
The trace minerals known to be essential for humans are iron, zinc, iodine, selenium, copper, manganese, fluorine, chromium, molybdenum, and cobalt. Others that may also be essential are arsenic, silicon, nickel, boron, tin, and vanadium. The list grows as we learn more about minerals.
Instead of providing detailed information on each trace mineral, we will look at their general characteristics such as functions, absorption, transport, and excretion; we will also examine their dietary sources. We cover iron and zinc in greater detail than other minerals. Finally, we will discuss the need for vitamin and mineral supplements.
This unit also covers the subject of blood health. This is mainly because the mineral that dominates this unit—iron—plays a major role in a key function of blood, namely the transport of oxygen. We also look at vitamin K, another nutrient with an important role in blood.
This unit consists of five sections:
12.1—Vitamin K
12.2—General Characteristics of Trace Minerals
12.3—Iron
12.4—Zinc
12.5—The Need for Vitamin and Mineral Supplements
12.6—Seeing the Whole Diet
Objectives
After completing this unit you should be able to
- identify the functions of vitamin K in the body, describe the symptoms of vitamin K deficiency and toxicity, and list some of its major food sources.
- classify the general functions of trace minerals in the body.
- briefly describe the absorption, transport, and excretion of trace minerals, and identify their general sources for dietary intake.
- describe the distribution and functions of iron in the body.
- briefly describe how iron is absorbed, transported, stored, and recycled in the body, and explain how the body maintains iron balance.
- describe the symptoms of iron deficiency, distinguish between iron deficiency and iron deficiency anemia, and identify the biochemical tests used to determine iron deficiencies and anemia.
- describe the causes and effects of iron overload.
- describe the forms of dietary iron and their sources, and identify the dietary factors that enhance and inhibit iron absorption.
- identify the major functions of zinc in the body, describe the causes and effects of zinc deficiency and zinc toxicity, and list some major dietary and non‑dietary sources of zinc.
- discuss the need for vitamin and mineral supplements.
12.1 Vitamin K
Introduction
The name vitamin K is based on the “koagulation” factor that was first discovered to be missing in the fat‑free diet fed to newly hatched chicks. These chicks developed hemorrhagic lesions on various parts of their bodies. When alfalfa or hog liver fat was given to these chicks, the hemorrhagic condition was reversed.
The unique feature of this vitamin is its synthesis in the human GI tract by micro‑organisms. When the gut flora are functioning normally, sufficient vitamin K is synthesized to meet as much as half of adult human needs.
Objectives
After completing this section, you should be able to
- identify the functions of vitamin K in the body.
- describe the prevalence of deficiency and its associated symptom.
- describe the toxicity symptoms.
- list some of its major food sources.
Key Terms
After completing section 12.1, you should be able to define and use the following terms in context:
vitamin K
hemorrhagic disease
Reading Assignment
- Chapter 14: “About Blood” and “Vitamin K,” pages 451–453
Vitamin K
Function
The primary role of vitamin K is in blood clotting. It is involved in the synthesis of blood‑clotting proteins, one of which is prothrombin. It is also involved in synthesis of bone proteins.
Deficiency
Primary vitamin K deficiency in humans is rare because of intestinal vitamin K synthesis. Mixed diets contribute more than adequate amounts of vitamin K, and the vitamin is stored in the liver. However, vitamin K deficiency may be seen in
- people on long‑term antibiotic therapy;
- newborn infants; and
- people suffering fat malabsorption caused by bile obstruction or pancreatic insufficiency.
The symptom of vitamin K deficiency is very specific—the inability of blood to clot. Upon injury, hemorrhages can occur internally as well as externally, and if vitamin K is severely deficient, uncontrolled bleeding can lead to death.
Sources
Major food sources of vitamin K are green leafy vegetables, such as spinach, kale, turnip greens, cabbage, and broccoli. Dairy products, eggs, and whole grains are also good sources. Most fruits and non‑leafy vegetables are poor sources, as are highly refined foods.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
12.2 General Characteristics of Trace Minerals
Introduction
A discussion of trace minerals emphasizes again that each nutrient has an optimum effect only at a particular level of intake. That too much or too little of a nutrient may be equally harmful is particularly true of trace minerals. Because of the minuscule amounts required, each trace mineral has a rather narrow range of safe intake, outside which lies a real possibility of deficiency or toxicity; many body systems might be affected.
Unlike excesses of water‑soluble vitamins, excesses of trace minerals are not excreted efficiently but tend to accumulate in tissues. Consequently, the best way of obtaining safe and adequate amounts is through a balanced diet rather than through supplements. Trace minerals are widely available in foods and in the environment.
Note: There are no Key Terms for this section.
Objectives
After completing this section, you should be able to
- classify the general functions of trace minerals in the body.
- briefly describe the absorption, transport, and excretion of trace minerals and identify their general sources for dietary intake.
Trace Minerals
Trace minerals are involved in many enzyme and hormone systems; they are also constituents of some body compounds. Their functions can be classified into two general categories:
- catalytic roles. Many trace minerals serve as cofactors for enzymatic reactions. As coenzymes, they work with enzymes to facilitate chemical reactions. An example is zinc, which functions in DNA and RNA polymerase during cell division and growth.
- structural roles. Some trace minerals are integrated into the structure of specific molecules or types of tissue. Since many of these molecules or tissues have regulatory functions, the trace mineral exerts its effect in this manner. Examples are given in Table 12.1 below.
Table 12.1 Functions of trace minerals integrated into molecules
| Mineral | Molecule or Tissue | Function |
| iodine | thyroxin | regulation of energy metabolism |
| iron | hemoglobin | oxygen transport |
| cobalt | vitamin B‑12 | transfer of 1‑carbon group |
| zinc | insulin | glucose utilization |
| fluoride | fluorapatite | strengthening of teeth |
Some trace minerals, such as iron and zinc, function both as catalysts and as structural components.
Absorption
The absorption of trace minerals is generally regulated at the mucosa of the small intestine. It depends greatly on the physiological need—that is, if more of a mineral is required, more will be absorbed from the diet. Oxalic and phytic acids can interfere with the absorption of trace minerals by binding with them to form insoluble complexes. Nutrient interactions can also affect the absorption of trace minerals. For instance, an excessive iron intake can depress the absorption of zinc, while large amounts of zinc can interfere with copper absorption.
Transport
The trace minerals are transported by binding to protein carriers, which may be specific (e.g., transferrin for iron) or general (e.g., albumin). Specific protein carriers are usually only about 30% saturated; the remaining capacity is reserved to buffer excesses of the minerals. After this buffering capacity is exhausted, toxicity results.
Excretion
The excretion of trace minerals—if any—is generally through feces, urine, shed cells, bile, and menses. Some losses may occur in sweat and breath, especially in hot climates.
Dietary Sources
The amounts of trace minerals present in plants depend, to some extent, on the mineral content of the soils in which the plants are grown. Soils deficient in a mineral may produce plants containing low amounts of that mineral; this is particularly true for iodine and selenium. As a rule, however, our foods come from many different geographical areas, so a balanced mixed diet should provide adequate amounts of all the essential trace minerals. Foods of animal origin, such as eggs, meat, fish, milk, and seafood, are likely to provide consistent and adequate amounts of trace minerals as their concentrations in such foods are relatively high and they are more readily absorbed.
In grains, trace minerals are usually concentrated in the outer bran coating and in the germ of whole grains. Milling of whole grains to produce refined white flours removes a significant portion of trace minerals from the diet. Boiling foods in large amounts of water and then discarding the water will lower trace mineral content through leaching.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
12.3 Iron
Introduction
Among the trace minerals known to be essential for humans, iron and zinc are probably the most investigated. Iron deficiency is the most common nutritional deficiency in North America and worldwide, affecting all socio‑economic groups. Infants, children, and women in their childbearing years are primarily affected, though some men also show signs of iron depletion.
Objectives
After completing this section, you should be able to
- describe the distribution and functions of iron in the body.
- briefly describe how iron is absorbed, transported, stored, and recycled in the body, and explain how the body maintains iron balance.
- describe the symptoms of iron deficiency, distinguish between iron deficiency and iron deficiency anemia, and identify the biochemical tests used to determine iron deficiencies and anemia.
- describe the causes and effects of iron overload.
- describe the forms of dietary iron and their sources, and identify the dietary factors that enhance and inhibit iron absorption.
Key Terms
After completing section 12.3, you should be able to define and use the following terms in context:
| metalloenzyme | hemosiderin |
| ferrous iron (Fe++) | transferrin saturation |
| ferric iron (Fe+++) | iron‑deficiency anemia |
| cytochromes | microcytic hypochromic anemia |
| hemoglobin | pica |
| myoglobin | hemochromatosis |
| heme iron | MFP factor |
| ferritin |
Reading Assignment
- Chapter 14: “Iron,” pages 453–462
Forms and Functions
Present in all cells of the body, iron plays an important role in many biochemical reactions. Three to five grams of iron are distributed throughout the body of healthy adults. Of this, about 70–80% is found in hemoglobin with the remainder in myoglobin, body stores, and iron‑containing enzymes. The major storage sites of iron are the liver, the spleen, and the bone marrow.
The iron in the body can be divided into two forms:
- Functional iron serves a metabolic role (in hemoglobin and myoglobin) or an enzymatic role (in enzymes containing iron as cofactor).
- Stored iron is found in ferritin and hemosiderin.
Ferritin is a complex consisting of a protein and iron; it is the chief temporary storage form for iron. It is present in the intestinal mucosa, the liver, and other organs.
Hemosiderin is a much larger insoluble molecule formed when the intake of iron is high; it is a long‑term storage form, and the release of iron from hemosiderin is much slower than from ferritin. Hemosiderin is present in the liver, spleen, and bone marrow.
Functions of iron:
- Oxygen transport from lungs to body tissues. As a component of hemoglobin in the red blood cells, iron combines with oxygen in the lungs, where the concentration is high, and is released in tissues, where the concentration is low. Muscle myoglobin picks up the oxygen and holds it for use during muscle contraction.
- Cellular respiration during the process of energy production. Iron is a constituent of the cytochromes, which are enzymes involved in the electron transport chain. Cytochromes are present in the mitochondria of all cells. Iron can switch back and forth in its two ionic states during oxidation‑reduction reactions. Through oxidation‑reduction reactions, energy is continuously generated for the normal functioning of the cells. A deficiency of iron therefore produces the symptoms associated with lack of energy, which we discuss later.
- Other functions. Iron also functions in biochemical reactions that are not directly involved in energy production. Some of these functions are connective tissue synthesis, antibody production, synthesis of purines found in DNA and RNA, and synthesis of brain neurotransmitters.
Absorption and Metabolism
The textbook presents a concise description of the absorption, transport, storage, and recycling of iron in the body. Although iron is never intentionally excreted, it is lost in many ways. It is present in minute amounts in every cell; thus, iron loss accompanies cell loss. The flaking of skin, the sloughing of mucosal cells from the intestine, and the loss of hair and nails result in iron loss. Urine, sweat, and intestinal secretions excreted in feces also contain iron. Blood loss represents a significant iron loss because most of the body’s iron is found in hemoglobin. Therefore, hemorrhages or blood donation can result in a major loss of iron—though hemorrhages, of course, seldom occur in healthy adults. Women, however, experience significant iron loss monthly through menstruation; the amount lost is highly variable, but the average is about 0.3 to 1.0 mg per day. Besides replenishing the daily loss of iron, extra iron is needed during cellular growth. Thus, infants, children, adolescents (especially girls), women in their childbearing years, and pregnant women have higher iron requirements and are more prone to iron deficiency.
The absorption rate of iron can range from 2–35%, with an average of 18%. The most influential determinant of iron absorption is the iron status of the individual—that is, the amount of iron present in body stores as ferritin (in the bone marrow, the liver, and the spleen). When the requirement increases, iron is initially withdrawn from stores. The rate at which iron is released from ferritin into the general circulation is regulated by the degree of transferrin saturation in the blood. Transferrin is the protein carrier required for iron transport in the blood, and is usually about 30% saturated when iron stores are adequate. The remaining transferrin circulates in the blood without iron. When iron stores become low, transferrin saturation decreases, which signals the mucosal cells to absorb more iron from the gut.
When intake has been high and stores are above 30% saturated, absorption drops, leaving the large majority of iron to be excreted in the feces. At the same time, the ferritin in the mucosal cells is also lost with the sloughing of cells. The net effect is to reduce the amount of iron entering the plasma. Figure 12.1 illustrates how normal, low, and high iron stores influence iron absorption.
Figure 12.1 Effect of iron stores on iron absorption
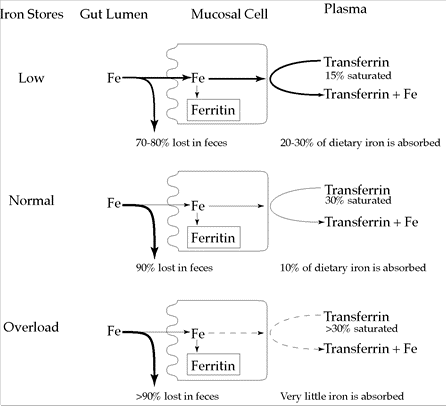
It should now be apparent that the site for control of iron balance is the gut; the stimulus to adjust the controls is the physiological need for iron (as reflected by body iron stores); and the mediator for control is transferrin saturation.
Deficiency and Toxicity
The body is usually able to maintain a normal iron balance. However, a consistently low intake of iron coupled with high iron requirements can exceed the body’s ability to maintain a balance. Body stores will eventually become depleted, causing hemoglobin levels to drop. This results in iron deficiency anemia, which is described as microcytic hypochromic anemia. Deficiency symptoms of iron are described in the textbook (see the summary, p. 462).
Iron deficiency can be described as progressing through two stages. The first stage is known simply as iron deficiency—that is, when iron stores are depleted. About 20% of women and 3% of men in Canada and the United States have iron deficiency. As pointed out in the textbook, people can be iron deficient without being anemic. This state is known as a subclinical deficiency. Sensitive biochemical tests, however, can identify iron deficiency before hemoglobin levels actually drop. A direct test of iron stores is the measure of blood (serum) ferritin. Indirect tests, which involve the measure of blood transferrin and the iron bound to it, are total iron‑binding capacity (TIBC) and transferrin saturation, respectively. During the depletion of iron stores, the concentration of transferrin (i.e., the protein that transports iron) increases. When transferrin increases without additional iron, the percentage transferrin saturation automatically decreases. Conversely, the TIBC (i.e., the measure of the total capacity of iron that transferrin can carry) increases.
The second stage is severe iron deficiency. It occurs after iron stores are completely exhausted, when hemoglobin levels begin to drop. Biochemical tests to detect anemia include red blood cell count, hematocrit (i.e., the volume of red blood cells in a given volume of blood), and, more directly, the measure of hemoglobin. For women, if hemoglobin levels drop below 12 g per 100 ml, the result is iron‑deficiency anemia, characterized by small, pale blood cells. About eight percent of women and one percent of men have iron‑deficiency anemia. Appendix E (pp. E‑16 to E‑18) gives a more detailed explanation of the different biochemical tests and their meanings. (You will not be tested on the details of this material.)
Anemia can easily be treated by iron supplementation if iron deficiency alone is present. Nutritional anemia may also be caused by deficiencies of vitamins B‑6 and B‑12 and folate. A low‑protein diet may result in anemia, as can vitamin C deficiency since vitamin C promotes iron absorption and enables transferrin to release iron into tissues. A lack of vitamin E may result in hemolytic anemia. Since copper is required for hemoglobin formation, a deficiency of this trace mineral may also produce anemia.
The textbook gives a concise description of the two types of iron overload. Hemochromatosis is caused by a hereditary defect that generally results in permanent damage to such tissues as the liver, spleen, and heart. Hemosiderosis is caused by excessive ingestion of iron that results in acute poisoning (toxicity).
Dietary Sources of Iron
There are two forms of iron in foods: heme iron and non‑heme iron. Bound in hemoglobin and myoglobin, heme iron makes up about 40% of the total amount of iron in animal tissues. The remaining 60% is non‑heme iron and consists of ionized iron (Fe+++ or Fe++) or ferritin iron found in tissues.
The heme portion of the hemoglobin molecule is absorbed intact by the mucosal cells. Once inside a cell, iron is released, then transported across the cell to be picked up by the plasma transferrin and delivered to other body cells. Figure 12.2 illustrates the absorption of heme iron.
Figure 12.2 Absorption of heme iron
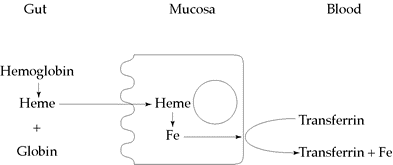
Heme iron is absorbed as a heme complex; therefore, it is not affected by enhancing or inhibiting factors in the diet. The absorption is relatively high, at a constant rate of about 25%. The major sources are meat, fish, and poultry.
The major part of dietary iron is non‑heme iron (ferrous and ferric). It is found in the non‑heme portion of meats, fruits, vegetables, cereals, eggs, dairy products, iron added to foods as part of enrichment programs, and iron found in mineral supplements. Non‑heme iron can become bound to binding agents such as phytates; thus, non‑heme iron has lower bioavailability than does heme iron. Non‑heme iron is absorbed at a lower rate than heme iron—about 2–20%, with an average of about 17%. Its absorption depends on the presence of enhancing or inhibiting factors. Another important factor is the iron stores; people with low iron stores absorb both heme and non‑heme iron more efficiently than people with normal iron stores.
The dietary factors that enhance or inhibit iron absorption are listed on page 454. Inhibition of iron absorption may also occur with the use of antacids, which cause precipitation of iron in the intestine as a result of the increase in pH. The occasional consumption of antacids is of no consequence to overall iron status. Very high phosphate intakes may also cause inhibition of iron absorption through the formation of insoluble iron phosphate.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
12.4 Zinc
Introduction
Zinc has many diverse roles in the body. The highest concentration is found in muscle and bone tissues. Although classic symptoms of severe zinc deficiency are not widespread in developed countries, marginal or mild zinc deficiency is quite common during adolescence, pregnancy, old age, times of stress, and disease.
Objective
After completing this section, you should be able to
- identify the major functions of zinc in the body, describe the causes and effects of zinc deficiency and zinc toxicity, and list some major dietary and non‑dietary sources of zinc.
Key Term
After completing section 12.4, you should be able to define and use the term dwarfism in context:
Reading Assignment
- Chapter 14: “Zinc,” pages 462–465
Note: You will not be tested on the details given in the section on zinc absorption and metabolism, or on Figure 14‑8 on page 463.
Functions
The main role of zinc is as the cofactor for over 100 enzymes. Some of the major functions are
- DNA and RNA synthesis. Zinc is required for growth, for wound healing, and, especially in males, for sexual maturation.
- vitamin A metabolism. Zinc is required in the mobilization of stored vitamin A from the liver when dietary vitamin A is low. Night blindness can result from zinc deficiency.
- insulin synthesis. Zinc is a structural component of insulin. It also seems to be involved in the storage and release of insulin.
- taste and appetite. Zinc deficiency can cause a decrease of taste acuity and loss of appetite.
- prevention of heavy metal poisoning. Zinc deficiency enhances both lead and cadmium accumulation and sensitivity to their effects.
- immunity. The immune system is particularly sensitive to a zinc deficiency.
Deficiency and Toxicity
Zinc deficiency is not widespread in developed countries, where meat is readily available. However, marginal deficiency resulting from unusual dietary practices may occur. The lack of a reliable index for detecting zinc deficiency makes the prevalence of marginal deficiency difficult to determine. Those susceptible to zinc deficiency are children who consume little meat and people who are pregnant, elderly, or poor. Symptoms of zinc deficiency are described on page 464 and summarized on page 465.
In children, toxicity can occur at two to three times the RDA. It can be caused by an overdose of a zinc supplement, or from storing acidic foods in galvanized cans. The principal toxic effect of zinc is its interference with normal copper metabolism (i.e., it tends to deplete the body’s copper level). As a result, it causes heart muscle degeneration, increases low-density lipoprotein, and decreases high-density lipoprotein in the blood. Each of these effects can accelerate atherosclerosis. Symptoms of zinc toxicity are summarized on pages 464 and 465.
Dietary Sources
The rich dietary sources of zinc are protein‑rich foods, such as meats, poultry, liver, and shellfish (e.g., oysters and crabs). Two servings of animal proteins per day can provide enough zinc for a healthy individual.
Although phytates and fibres in whole grains and legumes may inhibit zinc absorption, these foods are also rich sources of zinc. Consequently, in normal diets there should be no concern about inhibition, except in some vegetarian diets. However, high intakes of iron and copper may impair zinc absorption. At the same time, glucose or lactose in the diet can enhance zinc absorption. Zinc is better absorbed from human milk than from cow’s milk. Red wine seems to increase zinc absorption as well as iron absorption.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
12.5 The Need for Vitamin and Mineral Supplements
Introduction
About half of the adult population uses vitamin and mineral supplements on a daily basis. People take supplements as a form of health insurance, just in case their food intake does not provide adequate nutrients. For some people, vitamin and mineral supplements may be rational and beneficial; for others, the supplements are unnecessary and a waste of money.
In this concluding section, we look at the population groups at risk of developing vitamin and mineral inadequacies, present arguments for and against the use of supplements, and provide some guidance to selecting a “safe” vitamin and mineral supplement.
Objective
After completing this section, you should be able to
- discuss the need for vitamin and mineral supplements.
Key Term
After completing section 12.5, you should be able to define and use the following term in context:
subclinical deficiency
Reading Assignment
- Chapter 10: “Highlight 10: Vitamin and Mineral Supplements,” pages 354–359
Vitamin and Mineral Supplements
Vitamin and mineral supplements are not usually necessary for people who eat a well‑balanced diet. In fact, the 50 or more necessary nutrients for the daily needs of the body can only be provided through food. Even the best supplement does not contain the substances present in natural foods that are believed to possess health‑promoting benefits but which have not been properly characterized (i.e., phytochemicals). None of the essential macronutrients—carbohydrates, lipids, and proteins—are included in vitamin and mineral supplements. Above all, we eat foods—not nutrients—to live.
One common reason reported for supplement use is concern that the produce available in the food supply is inadequate in nutrients. You have already learned that the nutrient content of foods can vary for a number of reasons (see Unit 1, section 1.2). By far the major reason is eating a diet with a large content of highly processed food, such as sugar and white bread, rather than whole grains, fruit, and vegetables. However, there is no evidence that the fruits and vegetables in our food supply have an inadequate content of nutrients.
The textbook provides an informative account of the situations where supplements may be justified, of the potential hazards of using supplements, and of some of the misleading claims often used to increase sales.
A major survey conducted in 2004 of Canadians’ diets revealed that a large number of people is not reaching the RDA for various nutrients. Problem nutrients included vitamins A, C, and D, folate, calcium, and magnesium (Shakur et al., 2012). As explained above and in the textbook, the best solution to this problem is for people to make an extra effort to consume a healthy diet. However, for some groups this may not be practical. Problem groups include those with a long‑term low energy intake, alcoholics, and many elderly people. For people who fall into groups such as these, a vitamin‑mineral supplement is often justified.
Does taking a supplement lead to improved health for the general population? The answer appears to be a clear no. Our best evidence for this comes from studies that have examined whether use of vitamin‑mineral supplements reduce the risk of death. Five cohort studies followed large groups of adults, usually aged between 50 and 70, for long periods (8 to 10 years) and reported that total mortality (the risk of death from any cause) was no lower among regular users of multivitamins than among non‑users. These findings are supported by the results of randomized controlled trials. Several such trials have taken place to determine whether supplements have any value in the areas of all‑cause mortality, cardiovascular disease, cancer, or cognitive impairment. The findings have been clearly negative (Guallar et al., 2013).
Therefore, a more targeted approach to taking a multivitamin makes more sense than a blanket recommendation that everyone should take one. First, multivitamins should only be recommended for specific groups, as suggested above. Second, specific supplements should only be recommended for specific groups. Here are some examples:
- Folic acid (i.e., the form of folate used in supplements) is used for women of childbearing age to prevent neural tube defects in infants that such women may bear. This vitamin has been added to grains since 1998 in both Canada and the USA. As a result, the incidence of neural tube defects has fallen sharply in both countries.
- Vitamin D supplements may be valuable for people in northern latitudes during the winter. As discussed in Unit 11 (section 11.2), there is now considerable evidence that people who live in northern latitudes—including all of Canada—are usually marginally deficient in vitamin D by spring. This increases the risk of cancer. Supplements of vitamin D are now recommended for Canadian adults.
- Iron supplements may be required by women with high menstrual blood loss.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
12.6 Seeing the Whole Diet
This course has focused on the individual substances present in food, such as fibre, protein, vitamins, and minerals. By learning about these topics you can understand the science behind nutrition, namely, the role of these substances in the body. In many cases, we can understand health and disease in relation to individual substances; for example, the role of sodium in blood pressure, iron in anemia, vitamin D in bone health, and trans fats in heart disease. However, a major trend in modern nutrition is to look at foods while paying much less attention to the individual substances in the food. Going further, the diet is seen as a whole is a focus of attention.
Meat is a major source of protein, saturated fat, and iron. But meat has important health effects that go beyond these substances. Many studies have reported that a diet with a relatively high content of meat is associated with an increased risk of certain diseases. This is hard to explain in terms of the substances contained in meat. By contrast, when the diet has a generous content of whole grains, there is a lower risk of several diseases. This is probably related to the fact that whole grains are rich in fibre and phytochemicals as well as various nutrients such as potassium, folate, and magnesium. Accordingly, dietary advice is based on recommending a healthy dietary pattern, which includes less meat and more whole grains. This is consistent with what you read in Unit 2 about diet‑planning guides.
These concepts are covered in more detail in the follow‑up course, which is discussed below.
Looking Ahead to The Next Course
Now you have almost completed this course you should carefully consider registering in Athabasca University’s Nutrition 405: Nutrition in Health and Disease. That course builds on this course and explores exciting areas of modern nutrition with a major focus on nutrition and chronic diseases, including cardiovascular disease, cancer, and obesity. The course also covers nutrition in relation to exercise and nutrition through the lifecycle.
References
Guallar, E., Stranges, S., Mulrow, C., et al. (2013). Enough is enough: stop wasting money on vitamin and mineral supplements. Annals of Internal Medicine, 159: 850–851.
Shakur, Y. A., Tarasuk, V., Corey, P., & O’Connor, D. L. (2012). A comparison of micronutrient inadequacy and risk of high micronutrient intakes among vitamin and mineral supplement users and non‑users in Canada. Journal of Nutrition, 142: 534–540.
