Nutrition 330 Introductory Nutrition
Study Guide: Unit 7
Metabolism of Nutrients and Energy Balance
After carbohydrates, lipids, and proteins have been digested and absorbed, their breakdown products are transported to the liver via the bloodstream, and then to the body cells for metabolism. The term metabolism comes from the Greek word metaballein, meaning “to change.” It refers to all chemical reactions that go on in living cells. The metabolism of energy‑yielding nutrients involves two main processes: anabolism and catabolism. Anabolism is the building of complex body compounds from simpler substances; catabolism is the breaking of large molecules into smaller constituents. When anabolism exceeds catabolism in the body, growth, or weight gain, occurs. Conversely, if catabolism exceeds anabolism, loss of tissue substance, or weight loss, occurs. A balance between these two processes is necessary to maintain body tissues and body weight.
This unit provides an overview of the four major pathways for energy production and examines how the basic units of carbohydrates, lipids, and proteins enter these pathways. It analyzes the effects of feasting, fasting, a low‑carbohydrate diet, and a protein‑sparing fast on body metabolism. In the last section of the unit, we define the three major categories of energy expenditure and demonstrate how to estimate energy expenditure; we also review the common methods of assessing body weight and body composition and discuss the problems associated with overweight or underweight. Finally, we analyze the proper approach to weight control and identify criteria for evaluating weight‑loss diets.
This unit consists of three sections:
7.1—Metabolism of Nutrients
7.2—Energy Balance
7.3—Weight Control
Objectives
After completing this unit, you should be able to
- explain the role of adenosine triphosphate (ATP [chemical characteristics]) in the body.
- discuss the three main pathways involved in glucose catabolism: glycolysis, the tricarboxylic acid (TCA) cycle, and the electron transport chain.
- briefly describe the catabolism of glycerol and fatty acids in energy production, and explain why lipids are a rich source of energy but a poor source of glucose.
- briefly describe the catabolism of amino acids in energy production and explain how the body excretes excess nitrogen.
- describe the metabolism of carbohydrates, lipids, and proteins when energy needs have been met (while food energy is plentiful).
- describe the metabolic and physiological effects of fasting and low‑carbohydrate dieting.
- define ketosis and explain the condition that leads to its development.
- distinguish between direct and indirect calorimetry and between gross energy values and physiological values of foods.
- calculate the energy content of food and the percentage of energy derived from each macronutrient.
- identify the type of diet that provides the greatest satiety.
- define the three major categories of thermogenesis (energy expenditure), calculate basal metabolic rate (BMR), discuss the factors that affect BMR, and explain how energy expenditure can be estimated.
- describe the following common measurements used in assessing body weight or body composition: body mass index, skinfold measurement, and waist circumference.
- calculate a person’s BMI and interpret its significance.
- identify the health hazards associated with being overweight and underweight.
- discuss the factors that cause one to be overweight and obesity.
- describe the recommended approach to weight control.
- identify the shortcomings of a low‑carbohydrate diet.
7.1 Metabolism of Nutrients
Introduction
The three energy‑yielding nutrients that enter body cells for metabolism are glucose from carbohydrates, fatty acids from lipids, and amino acids from proteins. Depending on the body’s needs, these nutrients can be anabolized into complex body compounds (a process that requires energy) or catabolized into carbon dioxide and water (a process that releases energy). The four major pathways by which energy is produced are glycolysis, the tricarboxylic acid (TCA) cycle, beta‑oxidation, and the electron transport system. These pathways are interconnected and highly organized. The metabolism of nutrients is regulated by specific enzymes, coenzymes (many of which are B vitamins), cofactors (many of which are trace minerals), and hormones. Although vitamins and minerals do not yield energy, they act as essential facilitators for the liberation of energy from carbohydrates, lipids, and proteins.
Objectives
After completing this section, you should be able to
- explain the role of adenosine triphosphate (ATP [chemical characteristics]) in the body.
- discuss the three main pathways involved in glucose catabolism: glycolysis, the tricarboxylic acid (TCA) cycle, and the electron transport chain.
- briefly describe the catabolism of glycerol and fatty acids in energy production, and explain why lipids are a rich source of energy but a poor source of glucose.
- briefly describe the catabolism of amino acids in energy production, and explain how the body excretes excess nitrogen.
- describe the metabolism of carbohydrates, lipids, and proteins when energy needs have been met (while food energy is plentiful).
- describe the metabolic and physiological effects of fasting and low‑carbohydrate dieting.
- define ketosis and explain the condition that leads to its development.
Key Terms
After completing section 7.1, you should be able to define and use the following terms in context:
| metabolism | TCA cycle/Krebs cycle |
| energy metabolism | oxidative phosphorylation |
| anabolism | electron transport chain |
| catabolism | gluconeogenesis |
| ATP (adenosine triphosphate) | beta‑oxidation |
| glycolysis | urea |
| pyruvate | ketone body |
| acetyl‑CoA | ketosis |
| deamination |
Reading Assignment
- Chapter 7: Introduction and “Chemical Reactions in the Body,” pages 213–217
Energy Metabolism
Energy in food is trapped within the chemical bonds that hold molecules together. During catabolism of energy‑yielding nutrients, chemical bonds are broken. Most of the associated energy is transferred to chemical bonds of another molecule—adenosine triphosphate (ATP)—which is formed from adenosine diphosphate (ADP) and a free phosphate in the following reaction:
ADP + P (phosphate) + energy → ATP
The components of ATP are described in simple terms on page 216. The high‑energy phosphate bonds of ATP can be readily broken to release free energy that a cell can use:
ATP → ADP + P + energy for body cells
This instant energy is available to all cells for muscle work, growth, synthesis of body compounds, and other vital activities.
Reading Assignment
- Chapter 7: “Breaking Down Nutrients for Energy,” pages 217–223 (up to “Glycerol and Fatty Acids”)
Glucose Catabolism
Glucose is the primary fuel that body cells catabolize to produce energy. Although they are highly complex, the three main pathways of glucose catabolism are well understood; details can be found in Appendix C of the textbook. For this course, however, you need only understand the discussion below and recognize the overall significance of each pathway and of the important intermediates described in the textbook.
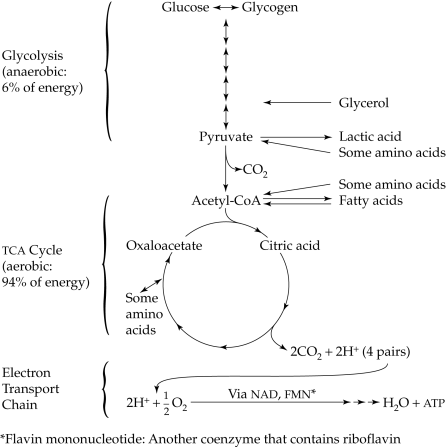
Glycolysis is the preliminary phase of glucose catabolism that takes place in the cytoplasm of the cell (see Appendix A for a discussion of cell structure). The overall result of this pathway is the splitting of glucose, a six‑carbon molecule, into two three‑carbon molecules of pyruvate. This pathway generates only about 6% of the total possible energy (ATP) yield from glucose. Because this pathway is anaerobic (does not require oxygen), it becomes an important source of energy production when oxygen is limited. This situation occurs during intense exercise, when oxygen cannot be delivered fast enough to the cells (e.g., during a sprint or in weightlifting). Under anaerobic conditions, pyruvate is converted to lactic acid instead of continuing into the next cycle that requires oxygen. The formation of lactic acid helps to buy time until oxygen again becomes available for complete breakdown. After a bout of anaerobic exercise, one must slow down and breathe deeply, to allow excess lactic acid in the muscles to be converted back to pyruvate. Alternatively, the lactic acid can be taken by the blood to the liver, where it is converted back to glucose. The glucose can then be used by the muscle cells to replenish glycogen stores or to produce energy aerobically again. If lactic acid accumulates in muscle cells, pain and fatigue result, especially in untrained muscles during heavy, sustained exercise.
The tricarboxylic acid (TCA) cycle is also known as the Krebs cycle or the citric acid cycle. This pathway is the second stage of glucose catabolism that takes place in the mitochondria of the cell. Unlike glycolysis, it requires oxygen. It involves a series of oxidation reactions, which generate about 94% of the total available energy from glucose. The preparatory step for this cycle is the oxidative decarboxylation of pyruvate by removal of a carbon from the three‑carbon molecule to form carbon dioxide and acetate (two‑carbon molecule). Acetate then attaches to a molecule of coenzyme A (CoA) to form acetyl‑CoA.
This reaction requires B vitamins, including thiamin, niacin, and pantothenic acid, which are essential components of the coenzymes TPP, NAD, and CoA, respectively. Riboflavin and vitamin B12 are needed in later steps of the TCA cycle. We discuss the B vitamins further in Unit 8. Acetyl‑CoA is the “pivotal” point of the TCA cycle because it allows for any substance that can be converted to acetyl‑CoA to enter the cycle. By this means, for example, glucose, glycerol, fatty acids, and amino acids can be catalyzed for energy. The TCA cycle is the central cycle for energy production from carbohydrates, lipids, and proteins. However, since acetyl‑CoA cannot be reversed to re‑form pyruvate and glucose, certain amino acids (those that enter as acetyl‑CoA) and all fatty acids cannot be used to form glucose. A more detailed discussion is provided later.
The TCA cycle essentially begins when acetyl‑CoA combines with a molecule of oxaloacetate to form citric acid, the first intermediate that contains three carboxylic acid groups (which is how the names tricarboxylic acid and citric acid cycle originated). The cycle continues with the formation of several other intermediates until oxaloacetate is resynthesized and combines with another acetyl‑CoA to begin another round of the process. During each cycle, two molecules of carbon dioxide are produced from one molecule of pyruvate and are eliminated in the air expired by the lungs. Other products of the TCA cycle are four pairs of hydrogen ions: H+. During oxidation reactions, hydrogen pairs and energy are released. The hydrogen pairs are picked up by coenzymes, which act as hydrogen carriers. Two of the coenzymes are nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). They contain two B vitamins, niacin (in NAD) and riboflavin (in FAD).
The electron transport chain, also known as the respiratory chain, is the final stage of glucose catabolism, where energy ends up as ATP. As implied, the electron transport chain involves the transfer of electrons from one coenzyme to another in an oxidation‑reduction process. Some energy is released in each transfer. The energy is trapped in the ATP molecule as a high‑energy phosphate bond. Since these reactions require oxygen and involve the phosphorylation of ADP to form ATP, the name oxidative phosphorylation is given to the process. At the end of the electron transport chain, hydrogen is combined with oxygen to produce water. The overall breakdown of glucose can be represented in this reaction:
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy (ATP)
Figure 7.1 Summary of Energy Production from Glucose
Reading Assignment
- Chapter 7: “Glycerol and Fatty Acids,” pages 223–224
Glycerol and Fatty Acids
As illustrated in Figures 7‑12 and 7‑13 (p. 225), only a small portion of the triglyceride molecule (i.e., the 3‑carbon “backbone”—glycerol) can be converted to glucose through gluconeogenesis. The rest of the molecule (i.e., the fatty acids) cannot. Each fatty acid is enzymatically catabolized by combining with CoA, where the chain is cleaved sequentially at the second carbon (i.e., the beta carbon), forming multiple units of acetyl‑CoA. This process, called beta‑oxidation or fatty acid oxidation, is the other major pathway of energy production and is illustrated in Figure 7‑11 on page 224, and Figure 7.2 in the Study Guide. Note: C16 denotes palmitic acid with 16 carbon atoms.
Figure 7.2 Beta‑oxidation of Palmitic Acid
C16 + CoA → C14 + Acetyl‑CoA + 2 hydrogen pairs
C14 + CoA → C12 + Acetyl‑CoA + 2 hydrogen pairs
C12 + CoA → C10 + Acetyl‑CoA + 2 hydrogen pairs
Besides the acetyl‑CoA produced in each step of beta‑oxidation, two pairs of hydrogen atoms are also removed. These hydrogen ions are picked up by NAD and FAD, which act as hydrogen carriers in the electron transport chain in the production of ATP. The acetyl‑CoA then enters the TCA cycle and thereby generates more hydrogen pairs for ATP production.
The extraction of energy from fats (through beta‑oxidation and the TCA cycle) is totally dependent on the presence of oxygen. That is why aerobic exercise (e.g., walking or jogging) is recommended as an effective way to burn body fat for weight control. During anaerobic exercise (e.g., weight lifting or sprinting), fat catabolism is halted.
Of the total energy released when fat is oxidized, about a quarter comes through beta‑oxidation and three‑quarters through the TCA cycle.
Triglycerides from food or from stored body fat are an excellent source of energy as ATP, but are only a minor source of glucose, because most of the triglyceride molecule is made up of fatty acids.
Reading Assignment
- Chapter 7: “Amino Acids” and “The Final Steps of Catabolism,” pages 224–230
Amino Acid Catabolism
The primary role of amino acids is protein synthesis; they are catabolized only when
- there is insufficient energy or glucose;
- there is an excess of proteins for body requirements; or
- the proteins are of such poor quality that protein synthesis is curtailed.
Amino acids must be deaminated before they can enter the pathways of energy production or storage. Deamination is the enzymatic removal of the amino group from the amino acid; it results in the production of ammonia. Deamination requires vitamin B‑6, niacin, and riboflavin. The ammonia is combined with carbon dioxide by the liver to form urea, which is then excreted in the urine by the kidneys. Production of urea is important, as urea is far less toxic than ammonia. Urea synthesis is illustrated in Figure 6‑14 on page 190.
Depending on the chemical structure of the carbon skeleton of the amino acid, it can enter the metabolic pathways through pyruvate, acetyl‑CoA, or certain intermediates of the TCA cycle (see Figure 7‑14 on page 226). Only those amino acids that can be converted to pyruvate can provide glucose through gluconeogenesis. About half of the amino acids are potential sources of glucose. Unlike fat, protein is a fairly good source of glucose in the absence of carbohydrate. You should recall from Unit 6 that glucose is the primary fuel for the brain, red blood cells, and nerve cells. Glucose‑generating mechanisms keep these vital tissues supplied with fuel even when carbohydrate is not supplied to the body, as in fasting.
Reading Assignment
- Chapter 7: “Energy Balance” pages 230–236
Feasting and Fasting
As illustrated in Figure 7‑20 on page 233, surplus carbohydrates, lipids, or proteins can be converted to body fat for storage. The body is far more efficient at storing excess dietary fat as body fat than it is at converting excess carbohydrate or protein to body fat. In addition to carbohydrates, proteins, or lipids, alcohol can also contribute energy or be converted into body fat.
The textbook gives a concise description of the metabolic effects at progressive stages of fasting. You should recognize that body proteins from muscle and lean tissue start being catabolized when glycogen stores are depleted and no carbohydrates are available, which happens after several hours of fasting or low‑carbohydrate dieting. Although a fairly high intake of protein may be provided by a diet very low in carbohydrate, a high intake of protein cannot prevent loss of body protein.
Ketone bodies are normal metabolites of the body. At low levels of production, the body is capable of metabolizing the ketone bodies. However, during fasting or low‑carbohydrate dieting, production of ketone bodies accelerates with fat catabolism. The lack of carbohydrate stimulates fatty acids to break down faster than the body can properly handle. Acetyl‑CoA then begins to accumulate because of insufficient supply of oxaloacetate (primarily derived from pyruvate, of which the major source is carbohydrates); acetyl‑CoA cannot form citric acid in the TCA cycle. As a result, acetyl‑CoA fragments condense to form ketone bodies (see Figure 7‑21, p. 235), some of which can be used to fuel particular brain cells and the nervous system. When ketone bodies reach a high concentration in the blood and urine, the condition is known as ketosis. Ketosis also happens in people with untreated diabetes, when body cells are literally starved of glucose. A characteristic ketone body is acetone, some of which is excreted through the lungs, giving a rather sweet odour; this is often called acetone breath. Mild ketosis suppresses appetite, increases urine output, and sometimes causes nausea. Severe ketosis can cause acidosis (a decline in the pH of the blood) and loss of water from body tissues (to excrete ketones). Consequently, sodium and potassium become depleted, blood pressure drops, and death can result from the collapse of the circulatory system.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
7.2 Energy Balance
Introduction
In simple terms, energy is the capacity to do work. In food, energy exists within the chemical bonds that hold the atoms together. When food is burned, energy is released in the form of heat. In the body, the energy produced through catabolism of food eventually yields heat in two ways: first, as a by‑product of metabolism (released as heat), and second, as a by‑product generated after muscular work (kinetic energy), brain and nerve activity (electrical energy), and synthesis of body tissues (chemical energy).
Energy balance is the balance of energy input versus energy output (expenditure). To balance the energy equation, the energy expended must be met by an intake of energy from carbohydrates, lipids, and proteins (and alcohol, if present). If energy input is greater than energy output, the result is weight gain; similarly, if energy output is greater than energy input, the result is weight loss. This is the basic energy balance equation. Supermarket magazines and supplements from health-food stores often promise to make fat “melt away.” Fat only disappears when it is consumed by metabolism.
Objectives
After completing this section, you should be able to
- distinguish between direct and indirect calorimetry and between gross energy values and physiological values of foods.
- calculate the energy content of food and the percentage of energy derived from each macronutrient.
- identify the type of diet that provides the greatest satiety.
- define the three major categories of thermogenesis (energy expenditure), calculate basal metabolic rate (BMR), discuss the factors that affect BMR, and explain how energy expenditure can be estimated.
Key Terms
After completing section 7.2, you should be able to define and use the following terms in context:
| calorimetry | basal metabolism |
| direct calorimetry | basal metabolic rate |
| indirect calorimetry | resting metabolic rate |
| gross energy value | thermic effect of food |
| physiological fuel value | body mass index (BMI) |
| thermogenesis | satiety |
Reading Assignment
- Chapter 8: Introduction, “Energy Balance,” and “Energy In: The kCalories Foods Provide,” pages 249–253
Determining Energy Values of Food
As we have seen, during catabolism of energy‑yielding nutrients, oxygen combines with carbon and hydrogen to form carbon dioxide, water, and energy, which can be measured as heat.
Direct calorimetry is the actual measurement of energy as heat. As illustrated in Figure 8‑1, page 251, a bomb calorimeter directly calculates the energy in foods by measuring the total amount of heat released after complete combustion (burning) of a food sample. Direct calorimetry can also be used to measure the total amount of heat released by an actual person, from which energy expenditure can be calculated. This method involves a specially designed chamber and is expensive.
Indirect calorimetry is the measurement of oxygen consumption and carbon dioxide production by which to determine, indirectly, the amount of energy used by the body. It requires only a portable respiration apparatus, which is simpler, less expensive, and more mobile than the apparatus needed for direct calorimetry. It also allows for the measurement of a wider range of physical activities. Consequently, indirect calorimetry is preferred to direct calorimetry for measuring human energy expenditure.
The gross energy values of food are the amounts of energy determined by a bomb calorimeter when food is completely burned. In the body, however, energy extracted from food is less than its gross energy value. Through incomplete digestion, absorption, and metabolism, a small amount of food energy is lost, and is excreted in either the feces or the urine. By mathematical correction, gross energy values can be adjusted to better represent the amounts of energy available to the body. Such values are called physiological fuel values. These values are typically listed as calories or kilocalories in food composition data and on food labels. You have already learned that the physiological fuel values for carbohydrates, lipids, proteins, and alcohol are four, nine, four and seven kilocalories per gram, respectively.
Table 7.1 Energy Values of Food
| Energy nutrients | Gross energy values (kcal per gram) | Physiological fuel values (kcal per gram) |
| Carbohydrates | 4.10 | 4.0 |
| Lipids | 9.45 | 9.0 |
| Protein | 5.65 | 4.0 |
| Alcohol | 7.10 | 7.0 |
Hunger and Satiety
Hunger and satiety, when in harmony, allow people to eat the right amount to meet their energy needs while maintaining a normal, consistent body weight. The textbook briefly describes the influences that prompt us to eat and the factors that induce satiation (pages 251–253). Satiety is hormonally regulated but is also influenced by the nutrient composition of the diet. Of the macronutrients, protein has the greatest satiety value because it takes longer to digest than carbohydrate or fat, and helps to keep blood glucose levels constant. High‑fibre foods also provide satiety. Foods high in fat are weakly satiating, yet provide a concentrated source of calories. Thus, a small amount of protein, along with a source of fibre at each meal, may help to control appetite.
The textbook describes the concept of energy density. Fat is the biggest single factor that contributes to a high‑energy density. Foods with a high content of sugar or refined (white) flour also tend to have a high‑energy density. It is easy to consume high‑energy‑density food in excess before satiation causes eating to cease.
A crucial factor in satiety is water. Water dilutes food energy. Foods with a high water content, such as soup, fruit, and salad items, have a low energy density and, therefore, induce satiation after a relatively small amount of food energy has been consumed. A combination of a high content of both water and fibre, as in fruit and salad items, therefore gives food an especially low energy density.
Reading Assignment
-
Chapter 8: “Energy Out: The kCalories the Body Expends,” pages 254–257
Note: You will not be tested on the details in Table 8‑2 on page 256.
Energy Expenditure
The three major categories of energy expenditure are basal metabolism, voluntary muscular activity, and the thermic effect of food (energy expended by the body for processing food).
Basal metabolism, also known as basal metabolic rate (BMR), is defined as the minimum energy required to maintain vital life processes, as described in the textbook. The measurement of BMR is generally done first thing in the morning, under controlled conditions:
- when the subject is awake
- 12 to 15 hours after eating (i.e., in the absence of digestion or absorption of food)
- when the subject is lying quietly with complete physical and mental rest
- when the room is at a comfortable temperature (25°C)
- when the subject is wearing light clothing
Factors that affect BMR are listed in Table 8‑1 on page 255 and explained in the section titled “Estimating Energy Requirements” on page 257. Body composition has the most significant influence on BMR. BMR can be elevated with exercise. Certain types of exercise also build lean muscle mass, and the leaner the body tissue, the higher the BMR—another reason why exercise is emphasized in maintaining BMR for weight control.
Voluntary muscular activity (or physical activity) is the most variable category that can influence an individual’s total energy output. It is also the most difficult to measure and can account for great errors in determining energy requirements because there are virtually endless possibilities for types of activities and no table of values comprehensive enough to cover all of them. Also, the intensity or speed of activity varies greatly among people. Body weight is another important factor: heavier people use more energy for a given activity.
The thermic effect of food is the energy required to digest, absorb, transport, metabolize, and store nutrients from food. You can relate the energy expended for this purpose to the rise in body temperature after a meal is consumed. The thermic effect of food depends on the carbohydrate, fat, and protein components in the diet. In a mixed diet, an average of about 10% of total energy intake is estimated for thermic effect of food. High protein foods create the greatest thermic effect.
The textbook identifies the fourth component of energy expenditure as adaptive thermogenesis. This process is not well described for humans, and is normally not considered in estimating energy requirements.
You should be able to estimate energy requirements based on BMR and physical activity. One method is shown in the “How To” box on page 259. A simpler method is shown here:
The most accurate way of determining energy requirements is by direct or indirect calorimetry, so as to measure actual energy expenditure. Another fairly accurate way is by estimating average energy intake from food over a period of time when body weight and activity are stable, which approximates the daily energy requirement.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
7.3 Weight Control
Introduction
Normally, our intake of food is regulated by the hypothalamus (feeding centre) of the brain, which controls hunger and satiety. A need for food triggers the sensation of hunger; after eating, food accumulates in the stomach and creates a feeling of satiety. In this way, a steady energy balance between intake and output can be maintained.
Other elements are involved in determining eating behaviour; for example, genetic, cultural, environmental, psychological, behavioural, physiological, and neurological factors. Although interesting, these elements lie beyond the scope of this course.
Objectives
After completing this section, you should be able to
- describe the following common measurements used in assessing body weight or body composition: body mass index, skinfold measurement, and waist circumference.
- calculate a person’s BMI and interpret its significance.
- identify the health hazards associated with overweight and underweight.
- discuss the factors that cause overweight and obesity.
- describe the recommended approach to weight control.
- identify the shortcomings of a low‑carbohydrate diet.
Key Terms
After completing section 7.3, you should be able to define and use the following terms in context:
| waist circumference | overweight |
| skinfold measurement | underweight |
| obesity | metabolic syndrome |
| body mass index | percent body fat |
Reading Assignment
- Chapter 8: “Body Weight and Body Composition” pages 258–263
-
Appendix E: “Anthropometric Measurements,” pages E4–E13
Note: You will not be tested on the “How To” box on page 259 or the material from Appendix E.
Anthropometric Measurements
In Unit 1, we introduced anthropometric measures as one of the four components of a complete nutritional assessment. Now, we shall take a closer look at the three most common measurements used in assessing body weight or body composition: body mass index, skinfold measurement, and waist circumference. We also look at percent body fat.
Body Mass Index (BMI)
Body mass index (BMI), described on pages 260–261, is an anthropometric measure based on the relationship of body weight to height. A graph for calculating BMI values appears in Table 8‑5 (p. 260). BMI has a high correlation with body fat. An older method of indicating appropriate weight ranges for height is height‑weight charts; however, these are no longer used.
The textbook gives the BMI values that are used to define the different weight classes. As indicated in Figure 8‑5 (p. 260) obesity can be broken down into different classes. Studies indicate that the lowest mortality rates are associated with a BMI of about 20–25. The risk of health problems increases above or below a BMI range of 18.5–25. As the BMI rises, so does the risk of obesity‑related diseases like diabetes and cardiovascular disease. The use of the BMI is suitable only for people between ages 20 and 65 years. BMI values are not valid for pregnant or breastfeeding women, or for muscular athletes. Chapter 17 of your textbook provides BMI values for children and adolescents from 2–19 years of age (p. 575). Also see Figure 17‑9 on page 576.
Percent Body Fat
The textbook explains the value of BMI measurements, but also notes that they can be misleading. This is because the BMI tells us nothing about body composition. Measuring percent body fat overcomes this problem. This can be done by hydrodensitometry and by bioelectrical impedance (see Figure 8‑9 on page 264). These methods are not commonly used beyond specialist purposes such as research and athletic assessment.
A much simpler method for estimating the amount of body fat is to measure skinfold. While quick and simple, it provides only a rough approximation of the amount of body fat. It measures the thickness of a fold of skin by means of a special caliper at several different body sites. The two sites that are most commonly used are the triceps skinfold (back of the arm, midway between the top of the shoulder and elbow), and the subscapular skinfold (on the back, below and parallel to the shoulder blade). Since each site has a different normative thickness, the measure must be compared with its appropriate standard.
Waist Measurements
Health professionals are now using waist circumference as an indicator of health risks from high body‑fat levels. For men, waist circumferences of greater than 102 cm (40 inches) are correlated with an increased risk of disease; for women, risk increases when waist circumference exceeds 90 cm (35 in).
The waist circumference is measured at the narrowest part of the trunk, at the navel. The waist circumference provides an indication of fat stored in the abdominal area, also called visceral fat. So‑called “apple‑shaped” people have higher risks for chronic diseases such as type 2 diabetes, heart disease, hypertension, and breast cancer. Fortunately, abdominal fat is easier to lose through diet and exercise than fat stored in the hip area.
Waist circumference is an easy measurement to take, and research has confirmed its correlation to chronic diseases. Muscular people tend not to be identified as at risk using waist circumference measures. A disadvantage of waist circumference is that people may not accurately measure their own waists. With a little training, this may be overcome. Waist circumference measures will not accurately reflect risk for pregnant women.
Note: “Risk” is a descriptor used when weight may be a concern for health. The higher the risk, the more likely that health problems may develop (although there are no guarantees).
Reading Assignment
- Chapter 8: “Health Risks Associated with Body Weight and Body Fat,” pages 264–266
Overweight and Obesity
According to major surveys conducted in 2008, about 25% of Canadian adults are obese. In addition, about 37% of Canadian adults are overweight. In total, therefore, about 62% have a BMI above 25 (Public Health Agency of Canada and Canadian Institute for Health Information, 2011). The prevalence of obesity is similar in each gender, but men are more likely to be overweight (44% in men vs. 30% in women). The prevalence of obesity steadily rises with age and peaks at about age 60. The prevalence of obesity in Canada has doubled since the 1980s (see Figure on p. 128). This enormous epidemic of obesity is seen around the world.
Overweight and obesity are associated with the presence of many diseases, including coronary heart disease, diabetes (type II), stroke, arthritis (especially in the hips, knees, and lower spine), gout, respiratory problems, varicose veins, gallstones, and several types of cancer (colon, prostate, breast). The textbook also describes the concept known as the metabolic syndrome (p. 266). This condition is highly correlated with obesity, especially in the abdominal or upper region of the body, and is thought to be a strong predisposing condition to diabetes and cardiovascular disease. Key features of the metabolic syndrome include abnormalities in blood lipids such as high triglycerides and low HDL, high blood pressure, and insulin resistance, and the condition promotes inflammation in various body tissues. The measurement of waist circumference predicts the metabolic syndrome. The impact of obesity is difficult to measure accurately because these diseases are multifactorial and often mediated by associated abnormalities, such as hypertension, hyperlipidemia, and impaired glucose tolerance. However, there is no disputing that obesity has reached epidemic proportions worldwide, and that it dramatically affects healthcare costs in developed nations.
In our image‑conscious society, obesity also carries social disadvantages; furthermore, it increases the risk of accidents.
Reading Assignment
-
Chapter 9: “Environment,” pages 285–286
Note: You may also read pages 279–284 for interest but you will not be tested on this.
The Causes of Obesity
The textbook discusses how the environment around us has effectively created the epidemic of obesity. Recall the concept of energy density (Section 7.2 of this unit, under “Hunger and Satiety.”) We can illustrate the importance of this by comparing the following foods. Fruit and soup have a high water content and therefore a low energy density. For example, an apple provides 70 kcal, while a bowl of tomato‑vegetable soup (250 mL) has only 55 kcal. By contrast, pizza consists mainly of fat and carbohydrate but with little water; a slice of pizza delivers 250 kcal. The energy‑dense food (pizza) facilitates a large energy intake before the appetite has been satiated, whereas foods with a low energy density, such as apples and soup, will create a feeling of fullness after relatively little energy has been consumed.
It might appear that our environment has been carefully designed to maximize weight gain. For this reason, the basic cause of obesity is sometimes referred to as the obesogenic environment. The subject of the dietary causes of obesity is examined in more detail in other courses (Nutrition 405 and Nutrition 406).
Reading Assignment
- Chapter 9: “Weight‑Loss Strategies,” pages 291–299 (to Environmental Influences)
-
Chapter 9: “Highlight 9: The Latest and Greatest Weight‑Loss Diet—Again,” pages 310–313
Note: You may also read pages 286–291 for interest, but you will not be tested on this.
Treatments for Obesity
The textbook discusses the sensible approach to weight control involving diet and exercise. This strategy is as applicable to the avoidance of excess weight gain as to the treatment of obesity. Environmental influences and behaviours and attitudes are also involved in weight management, but this course focuses on the nutritional aspects of weight control.
The textbook suggests a reduced intake of fat as part of a weight‑loss strategy (Table 9‑3 on p. 293, and bottom section of p. 295). However, the supporting evidence for this is quite weak.
In recent years, low‑carbohydrate diets have become a popular method by which to lose weight. Low‑carbohydrate diets can produce dramatic weight loss in the first few days. This result is, however, mostly caused by a loss of glycogen and water. Results of studies carried out in recent years have indicated that low‑carbohydrate diets such as the Atkins Diet can be a little more successful than conventional calorie‑reduced diets, at least for the first four to six months. Three factors probably account for this finding. First, as many foods are eliminated from the diet, the dieter may find the remaining foods monotonous and may eat less of them. Second, protein tends to be quite effective at satisfying the appetite. Third, ketosis suppresses the appetite. Early on, most nutritionists assumed that diets low in carbohydrates would cause an increase in the blood cholesterol level because of the increased consumption of saturated fats. In fact, this does not happen, although the reasons are not clear. The success of the low‑carbohydrate diet seems to fade after six months, and by the one‑year mark, such a diet does no better than conventional diets.
Based on the evidence, low‑carbohydrate diets are not recommended, primarily because they exclude important parts of the diet, notably fruits, some dairy products, and grain products. As a result, they are likely to be low in fibre and essential nutrients.
The criteria for identifying unscientific (fad) weight‑reduction diets are briefly listed in the “How To” box on page 313. In general, a nutritionally sound diet should follow Canada’s Food Guide (Unit 2).
Reading Assignment
- Chapter 9: “Underweight,” pages 304–305
Underweight
A person may be considered underweight when he or she is 10% or more below ideal body weight. The following discussion refers mainly to people who are markedly underweight. Underweight can be as serious a medical problem as overweight, especially in adolescent girls. Approximately 5% of girls and young women between the ages of 14 and 24 have an eating disorder. Highlight 8, “Eating Disorders” (pp. 269–276) addresses this topic. You may wish to read this section for interest.
The causes of being underweight are as many as the causes of obesity:
- consumption of an insufficient quality and quantity of food;
- poor absorption and utilization of food consumed;
- wasting diseases, such as TB, AIDS, hyperthyroidism, and cancer, which increase basal metabolic rate;
- excessive physical activity, as in the case of athletes in heavy training; and
- psychological or emotional disorders.
Being underweight poses a variety of health problems, as it is associated with undernutrition. The health problems associated with being underweight include nutrient deficiencies, anemia, decreased resistance to infection and diseases, retarded growth, amenorrhea (cessation of menstruation), chronic fatigue, over‑sensitivity to cold temperatures, heart irregularities, hypotension, psychological problems, and complications in pregnancy.
Study Questions
Start the Study Questions to test your knowledge of what you just learned. The Study Questions will open in a new window or browser tab.
Note: The Study Questions are not marked and do not count toward your course grade. You may revisit the Study Questions at any time during the course.
References
Public Health Agency of Canada & Canadian Institute for Health Information. (2011). Obesity in Canada. Retrieved September 18, 2012, from https://www.phac-aspc.gc.ca/hp-ps/hl-mvs/oic-oac/assets/pdf/oic-oac-eng.pdf
